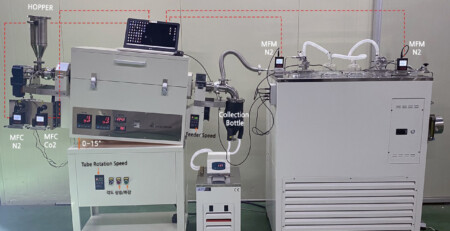
Rotary Tube Furnace for Battery Research
Rotary tube furnaces are integral to battery research, particularly the synthesis and processing of electrode materials.
Compared to stationary furnaces, rotary chambers offer three main benefits:
- Faster heat transfer within the sample.
- More even heat distribution.
- Continuous feeding capabilities.
After elaborating on the design of rotary tube furnaces, this article will use a recent patent as a case study of their role in cutting-edge battery research.
Rotary tube furnace technology, in brief
Rotary tube furnaces work similarly to a standard, fixed tube furnace. Both use a transparent, cylindrical, tubular chamber that’s indirectly (externally) heated by a series of heating elements underneath insulation.
(Directly fired furnaces, on the other hand, put a burner or heating element inside the chamber.)
They’re often paired with vacuum and gas flow management equipment to control atmospheric pressure and composition.
The main differentiators of a rotary design are the abilities a) to rotate the chamber axially for uniform heating and mixing, and b) to tilt it for continuous feeding.
Speaking of continuous processing, rotary tube furnaces also include a feeder at one end and a receiving vessel at the other end. Some materials are prone to clogging, so the feeder typically has its own screw conveyor and anti-clumping mechanisms.
As far as battery materials are concerned, rotary furnaces’ main benefit is to prevent stratification of samples via continuous mixing and consistent heating.
Visit this guide for a closer look at common applications of rotary tube furnaces and at some of our key safety and usability features.
Case study: cathode preparation from NMC powder
Global demand for large batteries is soaring, thanks largely to innovations in electric vehicles and stationary power plants. Battery fabrication carries high financial and environmental costs, so sustainability depends on greater efficiency and cycling stability that current techniques allow.
To that end, patent US11114662B2 shares a method and precursor for preparing nickel- (Ni-) based cathode materials for rechargeable lithium–ion (Li–ion) batteries.
The patent lays out the creation of a more sustainable option based on lithium–nickel–manganese–cobalt–oxide (NMC), which offers high energy density and structural stability. Thanks to lower use of scarce and costly cobalt, NMC is also more affordable than common alternatives like LiCoO2.
Technical summary
There are different compositions of NMC with varying degrees of excess nickel content, known as Ni–excess.
Broadly speaking, greater Ni–excess means greater energy density, so most researchers target “very high” or merely “high” levels.
Very high levels raise the risk of combustion, as delithiated (spent) cathodes essentially decompose and release oxygen.
High levels are less energy-dense, but also safer due to more stable oxygen-based compounds.
The applicants claim to have created a coating technique that reduces interactions between NMC and the cathodes, thus increasing the efficiency and lifespan of high Ni–excess NMC without the risk of very high Ni–excess formulations.
Their patented process includes a “double sintering” technique, roughly as follows:
- Start with a manganese (M)-based precursor derived from metal salts and a base.
- Mix the precursor with one of a few lithium-based compounds (LiOH, Li2O, or LiO.H2O) and sinter it in a rotary kiln (or rotary tube furnace) for ⅓–3 hours at 650–850° C. This yields a Li-deficient precursor powder.
- Mix the Li-deficient precursor with one of the lithium-based compounds mentioned above, then sinter that mixture for 6–36 hours at 800–1000° C. Now, the result is a positive electrode material.
- Combine the electrode material with various M oxides in one of three ways:
- Mix them, then sinter at 600– 800° C.
- Mix them with a fluorine polymer, then sinter at 250–500° C.
- Mix them with an inorganic oxidizing agent and an Li–acceptor, then sinter at 300–800° C (but most likely 350 to 450° C) in oxygen.
In all the above, external air flow through the furnace is generally 1.0–2.5 m3/kg. One exception involves an optional “roasting step,” wherein the M-based precursor is heated in N2 at 200+° C before mixing with a lithium compound.
They later compared the results of this rotary furnace process to more conventional techniques using a tray-based conveyor furnace.
From the authors’ 11 examples, below are three that we found particularly instructive.
Scenario 1: NMC preparation with double sintering
The first scenario, “Example 1,” demonstrates the use of double sintering to produce NMC with less lithium carbonate content and improved electrochemical performance.
- With LiOH.H2O as a lithium precursor, conduct initial sintering in a rotary furnace at 820° C for 2 hours.
- Conduct second sintering at 860° C for 10 hours in a tray furnace with continuous dry air flow.
Compared to the first step alone, the second step gave indications of less capacity loss per cycle and altogether improved performance.
Scenario 2: NMC preparation with roasted transition metal source & double sintering
The second scenario, “Example 2,” uses a roasted (calcined) transition metal source and double sintering to further enhance NMC’s cycle properties.
The process was similar to the above, but with preliminary roasting at 250° C in N2 prior to initial sintering. The second sintering was conducted at a marginally hotter 865° C.
Here, too, the double-sintered sample exhibited less lithium carbonate content and stronger indications of cycling stability.
Scenario 3: NMC sample with Al/sulfate coating
The last scenario, “Example 11,” introduces an Al/Sulfate coating on the NMC sample to further improve properties like discharge capacity and cycling stability.
The process was akin to the first scenario, with three significant modifications:
- The precursor was mixed nickel-manganese-cobalt oxyhydroxide.
- The sintered precursor had a lower Li:M ratio and a crystal size of 26.2 nm.
- Second sintering was lowered to 845° C.
To coat the double-sintered NMC product, the team blended it with Na2S2O8 and Al2O3, then heated it for a further 5 hours at 375° C.
As anticipated, the Al/sulfate coating enhanced discharge capacity and cycling stability more than double-sintering alone.
The microscopic images below show the texture of coated NMC particles. Note the relatively spherical particles, with even and consistent Al/sulfate distribution as a result of the heating process. This coating creates a higher capacity of ~210 mAh/g at up to ~3.4 V, as per the accompanying chart.

[Source: https://patents.google.com/patent/WO2020082019A1/en]
Why use a rotary tube furnace?
In each example, a rotary furnace played a key role in preparing lithium-deficient sintered precursors.
Its use consistently enhanced electrochemical properties, namely discharge capacity and cycling stability. The effect was magnified following double-sintering and coating. And given the large throughput of a rotary furnace, these processes are relatively scalable to meet commercial demand.
The applicants explain in detail their recommendation of a rotary furnace. We’ll set the complex chemistry aside for a moment to add context around their choice of equipment.
One overarching priority is homogeneous NMC. Practically speaking, that means every NMC particle has two things:
- A consistent ratio of manganese to lithium.
- A nearly identical degree of sintering.
Rotary tube furnaces achieve this by continuously moving and heating the material, which prevents sample stratification (layer formation), avoids delamination, and ensures uniform sintering.
Moreover, they do so with a compact footprint (compared to a rotary kiln) and high throughput. It’s often possible to increase production severalfold even without expanding facilities.
What sets SH rotary tube furnaces apart?
We covered our more universal innovations (like viewing ports, heating jackets, and clumping prevention mechanisms) in a separate article, linked earlier.
But a couple points are more specific to battery materials and precursors, so let’s take a moment to address them directly.
The patent’s authors note that rotary furnaces are suboptimal for the direct sintering of powders into solids. Their main concerns are corrosion (due to interaction with the chamber) and limited sintering times (due to the flow of materials).
These are valid issues, which we mitigate in a couple ways.
First, SH Scientific uses minimally reactive quartz chambers to reduce the likelihood of corrosion in general.
Unfortunately, lithium can react with silica (a component of quartz), so it’s impossible to eliminate all reactive potential for lithium-based compounds. That said, we offer several other materials for custom tubes optimized for specific samples or workflows.
Second, SH Scientific furnaces have adjustable tilt and rotational speed to enhance control over heating time.
This gives users the flexibility to choose the ideal rotational speed to mix the sample, then find an angle that slows passage just enough to extend sintering time.
Ordering your SH Scientific furnace
Our rotary tube furnaces translate decades of iteration and insights into unmatched precision, safety, and value.
And with our broad in-house customization capabilities, we’re already supplying teams with unique equipment that will make tomorrow’s battery technology possible.
Please contact our US sales office for technical inquiries, purchasing assistance, or a deeper discussion about your lab’s requirements.